Categories
Latest Posts
Running Cromwell on AWS/Batch
- Posted: 2018-11-21.
Parallel mysql myisam repair
- Posted: 2018-11-21.
Does a TKI like Crizotinib kill tumor cells ?
- Posted: 2018-10-28.
Save Spotify to flac or mp3
- Posted: 2018-07-14.
Replace all symlinks by the original file
- Posted: 2018-03-02.
Tag Collection
NSCLC Recovery cromwell javascript line-end Conky NFS spotify Paired-End api antialias Typesetting HPC bioinformatics docker ExecOnCommand Password preamble Searching compress Headless BASH osd_cat mysql torque/pbs python Ubuntu Apache TrainOfThought whitespace Yelo.tv fancy FTP apoptosis fuzzy match drmaa VMware Image indent VMplayer windows cotd AWS sudoers dos2unix natbib NGS timer R levenshtein spam Installation todo.txt telenet CPAN Silverlight mpd Perl Batch bibtex proftpd PHP Remote GATK Rcran Linespacing galaxy SLURM Cluster XFS Literature terraform cloudformation tikz LaTeX
Log in
Does a TKI like Crizotinib kill tumor cells ?
Posted on 2018-10-28 13:35:08
by Geert Vandeweyer
Loading Content
Does a TKI like Crizotinib kill tumor cells ?
Posted on 2018-10-28 13:35:08
by Geert Vandeweyer
When we were confronted with the diagnosis of Stage IV NSCLC, we were utterly shocked. How would you feel, when your wife, 31 years old, in good shape (we went on a 15km hike just 2 weeks before the diagnosis), was told she had advanced lung cancer? I'd guess you'd feel the same... "Luckily", an oncogene mutation was found, for which targeted therapy existed. The mutation, a ROS1 gene fusion, offered a better prognosis, with an average of 2 years progression free survival, using the recently approved Crizontinib drug.
As the biological role of ROS1 is not yet clear, nor is the exact mode of action of the drug, I decided to search the literature to find a reasonable answer. As it quickly became clear it wasn't fully understood yet, so I'd figure I might put my thoughts and understandings here, for the benefit of other patients, and for my own future reference. (note : you can immediately jump to the conclusion if you're not into biology :-))
So bear with me, as this my personal understanding of the scientific literature. I do not claim this to be correct !
1. Common questions
First, I came across the common questions and answers:
- Is crizotinib a cure for ROS1 cancer? No, it is an inhibitor of the cell growth, but doesn't kill the cells like classic chemotherapy or radiation
- How long do you take it? Until you develop resistance and the tumor progresses. All patients will develop this, as tumor cells are still present.
- If it doesn't kill the cells, why are some people NED (no evidence of disease). Why show many patients spectacular shrinkage of the tumor? Where did the tumor go? *silence*
I didn't understand this. And based on several online discussions, neither do a lot of other people. But in my opinion, there is an explanation, which is discussed below:
2. What are receptor tyrosine kinases
ROS1 is a so called receptor tyrosine kinase. Receptor proteins, located on the outside of cells, are responsible for transmitting environmental signals to the interior of the cell. These signals are responsible for events like cell growth, cell movement (migration) or cell death. Typically, molecules, like a hormone or a nutrient, bind to the outside part of two of these receptors. This binding brings both receptors together (forming what is called a dimer), leading to a change in its structure, and making the interior side available to be contacted by a "receptor tyrosine kinase". These RTK's will alter the interior part a bit, by adding a phosphate group to the "Tyrosine". This makes the receptor 'active', and passes on the signal. Simply put, a receptor protein can be seen as the first domino block in a domino series. The RTK can then be seen as the person pushing it, when the start signal is given, setting the whole series in motion. It's shown rather well in the video below.
In case of ROS1, the protein is both a receptor (it can bind to extracellular signal molecules), and a kinase. When the dimer is formed, ROS1 can add the phospate group to its own tyrosine residues on the intracellular part of the protein. This 'autophosporylation' capacity will become important later on.
2. Why do ROS1 gene fusions lead to cancer?
When this cascade is broken, it can lead to signal that constantly 'on'. Image a light switch you can't turn of. In case of ROS1 gene fusions, the inner part of the protein, with the signalling sections, is fused to a different exterior part. A part that doesn't need a external signal to push the domino series, or biologically, to create a receptor dimer. Hence, the protein keeps on firing signals, setting all kinds of cellular events into overdrive.
Although, the exact triggers and effects in healthy cells are not yet known for ROS1, it is known that the defective 'always ON' receptor, leads to increased cell growth and cell migration on one hand, and to reduced cell apoptosis on the other (source). In short, because ROS1 is always active, the cells keep on growing and won't die as they should. This is the definition of tumor cells...

3. Do we really not know which pathways are involved?
No, not really. But there are some hints and leads that helped me to understand the effect of Crizotinib. In the following sections, there are a lot of gene names mentioned. This might seem overwhelming, but keep in mind that the names don't really matter. Their function does: do they promote or inhibit growth, or do they cause cells to commit suicide or to ignore signals to die.
First, 20 years ago, it was shown by Zong et al that ROS1 is able to activate STAT3, a member of the JAK/STAT pathway (more on this later). They showed that increased STAT3 activation caused aberrant growth, independent of cell anchorage, and that blocking STAT3 function (by introducing dominant negative mutations that prohibit its functioning) reduces cell growth again. Thus, increasing ROS1 activity, as seen in ROS1+ cancer, causes cell growth, but only if the downstream actor STAT3 is functional.
A second study from 2002, by Nguyen et al showed that several members of another pathway, the PI3K-signalling pathway, were also involved in the aberrant growth following ROS1 overexpression, and that blocking these pathways blocks growth, even if ROS1 is still overactive.
So, it has been shown for at least two out of the 6 pathways in the picture above, that these are really relevant for the development of the tumor. Now let's move on to ROS1 inhibition...
4. What are tyrosine kinase inhitors ?
In medicine, or in biology in general, an inhibitor is something that blocks the normal function of a protein. When added to the cells, it disrupts the normal signalling flow in some way or another. You can image it as putting a heavy lock in the domino chain, which makes it impossible for the flow to continue:

In reality, tyrosine kinase inhibitors are very small molecules, that fit exactly into specific regions of the receptor proteins. You might visualize it like a lock and key principle. Because the TKIs bind very strongly to these regions, the natural binding partners cannot get access. In case of crizotinib, it binds the site where ATP normally binds when transferring the phospate group to the tyrosine positionin the ROS1 protein. Below, this interaction is shown with the ROS1 protein in blue, and Crizotinib as grey/green spheres:
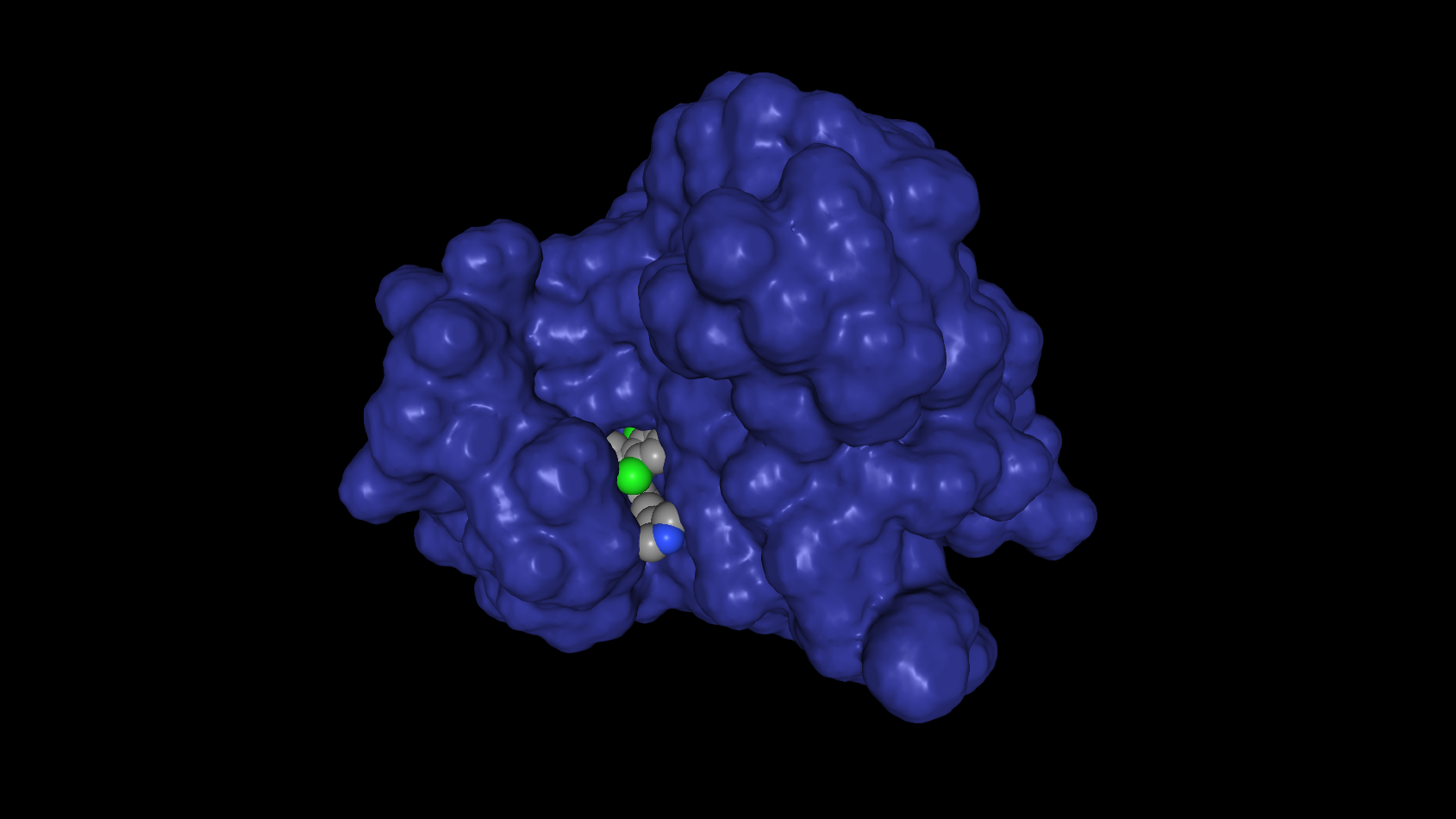
(warning : biology overkill) ATP is one of the energy sources available in cells. It consists of adenosine (one of the building blocks of DNA), with three (tri) phosphate groups bound to it (hence, (A)denosine (T)ri (P)hospate). By transfering one of these phosphates from ATP to various proteins, the receiving proteins become activated. They wake up and start performing their intended functions. The phospate transfer is mediated by a group of proteins called 'kinases', in case of receptor tyrosine kinases, they first transfer the phosphate to themselves, before activating other, downstream signalling proteins. It is this auto-phosphorylation that is blocked by Crizotinib, as the 'donor' ATP can not bind the required active site of the receptor. And as a consequence, the receptor cannot activate downstream signals.
So, Crizotinib is a very specific molecule that blocks the functioning of the ROS1 (and ALK) receptor tyrosine kinase. Overactive ROS1 is known to induce two processes involved in cell growth. So by shutting down the ROS1 signal, we are shutting down the continuous signal to grow. The light switch is 'ON', but we cut the wire. Being so specific, this also explains why mutations near the binding site of ATP can make the tumor resistant. If you slightly change the lock, one key, or in this case Crizotinib, won't fit, while another, ATP, might still do.
5. The role of STAT3 in NSCLC
All of the above explains why crizotinib stops tumor growth. We reduced (or eliminated) the signal to grow. This is what seems to be the general understanding amongst patients. Crizotinib halts excessive growth, but you need to keep taking the drug. The cells fall back to normal mechanisms, but they might keep dividing at a slow(er) pace, as lung epithelial cells seem te be capable of in the context of injury (source). This doesn't explain however, why many patients see a spectacular tumor reduction in the first weeks or months, with some patients even becoming NED (no evidence of disease, or clean scans). This seems to implicate that tumor cells are dying as a result of Crizotinib inhibition. So let's take another look at the pathways in which ROS1 is involved.
In the image above, there is a small box, labeled 'STATS', linking to proliferation and survival. Reality is slightly more complex, as the STATS pathway is one of the most essential systems in the body, regulating both cell growth, cell survival and cell death (source). In NSCLC specifically, the STAT3 gene is often known to be overexpressed. This overexpression is thought to be involved in excessive growth, ability to migrate and resistance to regulated cell death:
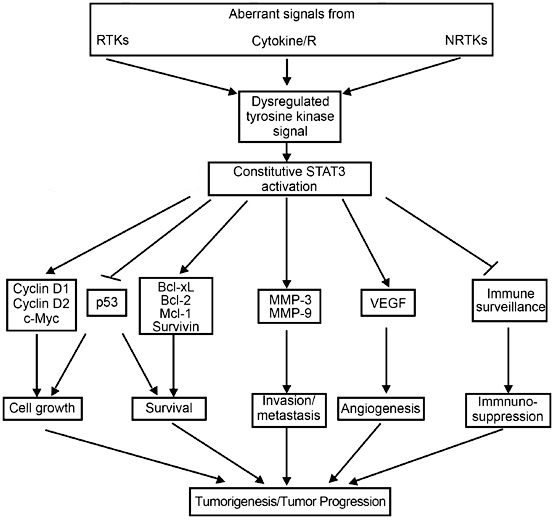
Legend: Arrows mean positive influence (promotes the function); forked lines mean negative influence (inhibits the function)
To explain tumor size reduction, it is important that ROS1 overexpression was shown to induce STAT3 (source), and recently, that Crizotinib lowers the STAT3 levels in an ALK+ cell line (H2228, source). Although the evidence is indirect (TKIs have not been shown to reduce STAT3 levels in ROS1+ NSCLC), it can be expected that lowering ROS1 levels will reduce the STAT3 overexpression. More importantly, the latter study report several interesing findings related to Crizotinib.
First, as expected, cell growth was strongly inhibited. Second, they also showed that crizotinib reduced cell migration, and that this inhibitory effect was enhanced with the prolongation of treatment. So not only growth rate is reduced, also the ability to metastasize seems to reduce over time. But most importantly, it was shown that the reduction of STAT3 increased the rate at which cells commit suicide, and the rate seems to increase over time.
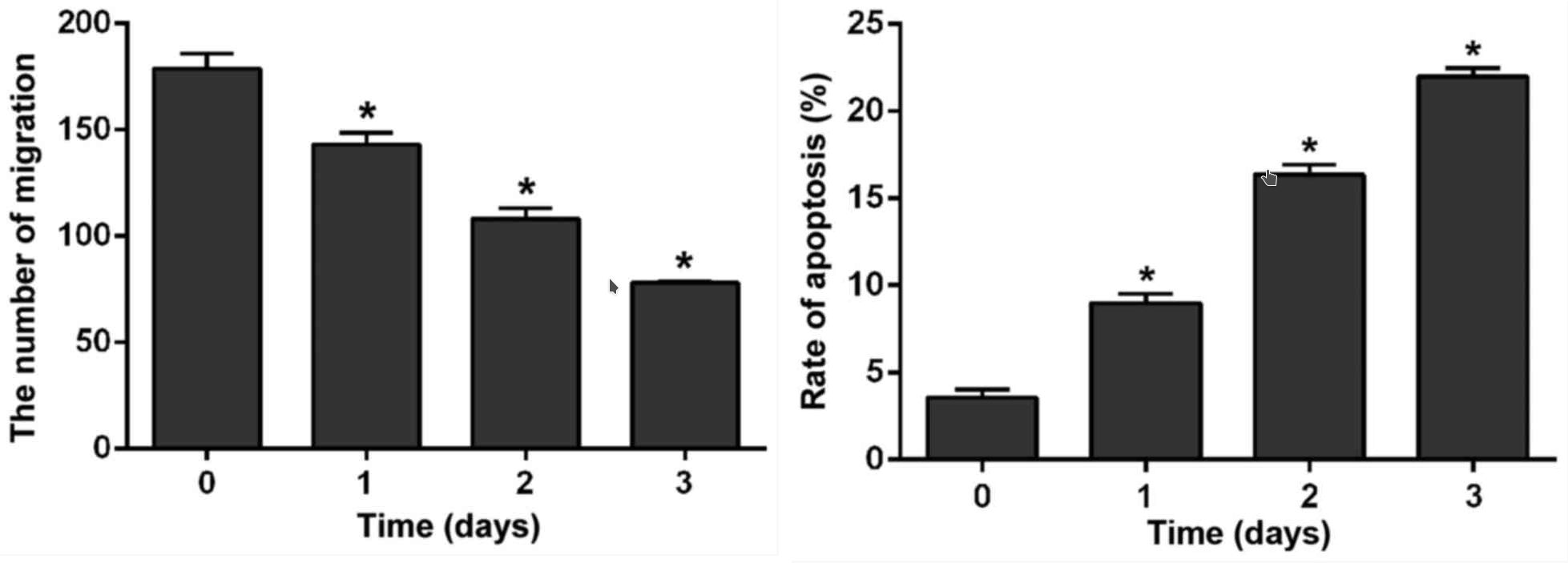
Left: Crizotinib reduces ability of cells to migrate. Right: Crizotinib increases the rate of cell death. Time represesents incubation time with Crizotinib. source
Although the exact mechanism of apoptosis is not mentioned in the article -and as a colleague pointed out, there are many, many more ways for a cell to die than I was aware of (see : here)-, it makes sense that the intrinsic pathway is involved, as STAT3 has been linked to it before. In brief (and not completely accurate), this mechanism causes cells to die in response to triggers inside the cell, such as DNA damage, becoming detached from neighboring cells or hypoxia.
This would means that high levels of STAT3 function as a brake on the cellular systems to clean up abnormal cells. In cancers, DNA damage is prevalant, as it helps the cancer cells to obtain more mutations, and become resistant to treatment. Also hypoxia is common, as the rapidly growing cells do not have sufficient access to blood vessels to get their oxygen supply. By releasing this brake, the suicide signals get the upper hand and the tumor cells die in massive numbers. As a result, we see a spectaluar tumor shrinkage.
6. Conclusions
In conclusion, we can say that ROS1 gene fusions causes a continuous signal to keep growing, while also suppressing the built-in system of cells to die if abnormalities arise. By releasing the brake on the latter system (mainly through the STAT3 pathway), we see a rapid decline in tumor size as highly abnormal cells are cleaned up. So does a TKI kill cells? No, but it allows cells to die, as they're supposed to do... Can you quit the TKI once your NED? Definitely now! Although the cell mass is gone, or has become too small to detect using PECT/CT, there is absolutely no guarantee that ALL tumor cells have died !
However, some questions remain (for which I don't know the answers):
Incomplete response: What if a fraction of the tumors remain? It might be that these cells are not 'abnormal enough', for example the core of the primary tumor, which really holds only the ROS1+ gene fusion. A single DNA rearrangment doesn't trigger apoptosis, as it can be seen in all cells of (healthy) people (source). On the other hand, they might have aquired other mechanisms to evade the regulated cell death mechanisms, through additional mutations.
Apply Chemo first? Quite a lot of the long time survivors in ROS1+ NSCLC received chemotherapy first, before starting TKI therapy. Correlation and causation are different things, I know, but it's striking. One explanation, and probably the correct one, is that TKI's were simply not available at the time of their diagnosis. Time will tell what fraction of people directly put on TKI's will reach these response times. However, considering the TKI-induced reactivation of cell death in response to DNA damage, it might make sense to wreak as much havoc on the cancer DNA as possible using chemotherapy, before starting the TKI. That's just a wild thought though...
One final conclusion would be that there is a lot to learn on the biology of ROS1+ NSCLC, and for this we need research. So please consider donating to the ROS1ders Cancer model project in the USA, or to Stichting Merels Wereld in Europe.
apoptosis, Literature, NSCLC, Searching, TrainOfThought
Comments
Dear Geert, came for the spotify software and stumbled upon this post. It looks like Crizotinib is considerably superior to classic chemo (https://pubmed.ncbi.nlm.nih.gov/32167664/). There are even better medications coming out, but they seem to be for very specific gene mutations. But you are probably googling this info yourself. Which your family the best! U. R
Comments
Loading Comments